Written by Michael Giedraitis – TPDDL Student Worker;
Edited by Hannah Ayala – Extension Assistant
Background
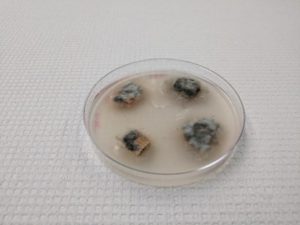
It was an exciting summer and fall at the Texas Plant Disease Diagnostic Laboratory. Alongside all our normal duties involved in operating the clinic, I was given the opportunity to work on the development of a new method for diagnosing oak wilt. Our current protocol is to break down infected branch samples sent to us and set them to incubate on petri plates in specialized media for two weeks. We check these plates for cultures of Bretziella fagacearum (formerly Ceratocystis fagacearum), the causal organism of oak wilt. Unfortunately, samples are often improperly shipped to the clinic, which can render the fungus inviable for growth on these plates. Even when samples are shipped properly, this method is susceptible to false negative diagnoses (33% efficacy).
New Techniques
Back in 2011, Dr. David Appel and Dr. Tom Kurdyla developed a set of primers and probe specific for PCR of B. fagacearum. Using these materials, Anna Yang and Dr. Juzwik of the University of Minnesota were able to demonstrate the efficacy of nested (100%) and real-time (87%) PCR assay for B. fagacearum in their oaks.
We attempted to adapt this protocol for diagnostic use in the clinic in order to save time and resources. 20 Texas live oaks were sampled for assay, and DNA was extracted with a variety of methods. We ran real-time PCR analysis to detect the presence of B. fagacearum in samples using the aforementioned primers and probe.

Picture of Michael holding reaction wells
Results
Unfortunately, our results for the PCR assay of B. fagacearum in Texas live oaks were not diagnostically useful. Several of the assay reactions demonstrated some amplification (which indicates presence of pathogen DNA) but were largely inhibited, probably by compounds endemic to the live oak tissue. This inhibition prevents a positive diagnosis being made, and thus prevents us from making use of this technique until it can be overcome.
Going Forwards
Even though we’ve been unable to adapt PCR assay for use in the diagnostic clinic, it remains a promising technique for the future. The ability to generate more reliable results in a fraction of the time is a powerful motivation for continued research. Currently we are investigating different methods of overcoming inhibition in the hopes of successfully adapting PCR assay for oak wilt diagnosis.
Want to learn more about Oak Wilt?
Visit texasoakwilt.org
WHAT Wednesday: Oak Wilt Sampling